
Taking BENDEKA
Your doctor will decide the appropriate dose of BENDEKA. As part of the treatment process, BENDEKA is recommended for intravenous infusion over 10 minutes on Days 1 and 2 of a treatment cycle, for 6 or 8 treatment cycles, depending on your condition.
BENDEKA may be given at a doctor’s office, the hospital, or an infusion center. It is given as an intravenous (IV) infusion that goes directly into a vein through a small needle in your arm.
The following 28- and 21-day calendars show which days during each treatment cycle BENDEKA will be given in either CLL or NHL and for how long.
CLL cycle of treatment
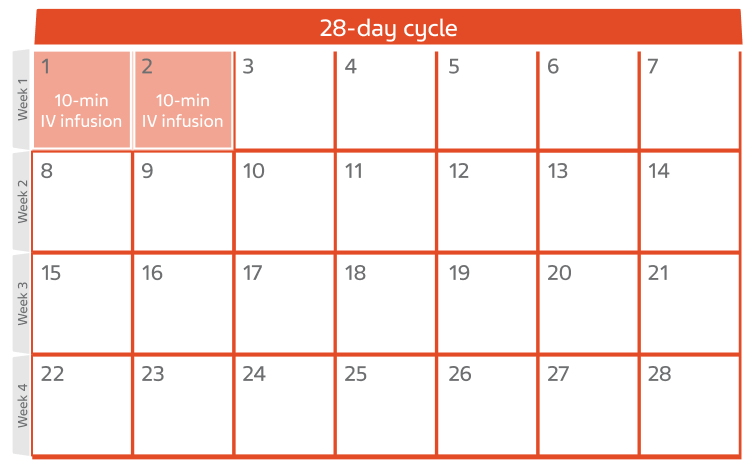
- This calendar shows 1 cycle of treatment for CLL with BENDEKA
- Each cycle lasts 28 days
- You will get your infusion on Day 1 and Day 2 of the 28-day cycle
- This treatment cycle may be repeated up to 6 times
NHL cycle of treatment
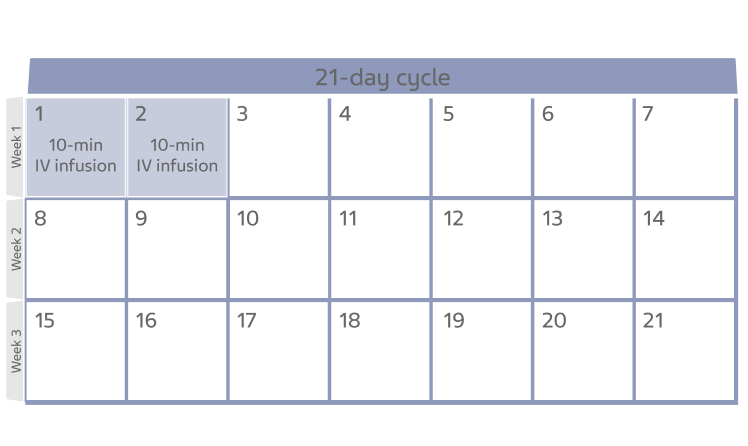
- This calendar shows 1 cycle of treatment for NHL with BENDEKA
- Each cycle lasts 21 days
- You will get your infusion on Day 1 and Day 2 of the 21-day cycle
- This treatment cycle may be repeated up to 8 times
Learn how often treatment with BENDEKA is given
You may or may not have already had an infusion with BENDEKA. Use this calendar tool to help you find out how often you’ll be given BENDEKA.
Select the information below to calculate how often you'll get your treatment and to see when other infusions will next occur. Calculate up to 6 infusion dates for CLL or 8 infusion dates for NHL.
-
- Cycles
- Day 1
- Day 2
-

CALENDAR

LIST
Your dose may change
Not all patients react to medications the same way, so it may be necessary for your doctor to make changes to the dose of BENDEKA to find out what is right for you or even to stop treatment. Changing the dose or delaying treatment may be necessary if you are experiencing side effects. The most important goal is to find the treatment approach that will help you achieve the best results possible. Your doctor may change, delay, or even stop your treatment.
Approved Use:
BENDEKA is indicated for the treatment of patients with
- ·Chronic lymphocytic leukemia (CLL). Efficacy relative to first-line therapies other than chlorambucil has not been established.
- ·Indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Important Safety Information
BENDEKA is not right for everyone,including patients with a known allergic response to bendamustine, polyethylene glycol 400, propylene glycol, or monothioglycerol.
BENDEKA may cause serious side effects including: low blood cell counts, infections or recurrence of infections, unexpected responses to BENDEKA when placed in your blood, sudden and severe allergic responses, kidney failure due to fast breakdown of cancer cells, other cancers, and leaking of BENDEKA out of your vein and into your surrounding skin. Some of these side effects, such as low blood counts, infections, liver injury, and severe allergic skin responses (when bendamustine HCl was given alone and in combination with other anticancer medications or allopurinol), have caused death.
Tell your doctor if you have any side effects including:
- -Signs of allergic reactions including; rash, facial swelling, or difficulty breathing during or soon after your infusion with BENDEKA injection.
- -Signs of infection including; shortness of breath, significant fatigue, bleeding, bruising, fever, or other signs of infection and or any suspicious skin changes.
- -Confusion, memory loss, trouble thinking, difficulty talking or walking, vision loss or other neurological or cognitive symptoms.
- -Nausea, vomiting, diarrhea, loss of appetite, or a yellow skin tone.
Some serious side effects may require changes in therapy, such as lowering the amount of BENDEKA given, stopping the use of BENDEKA, or waiting longer than expected between doses of BENDEKA.
BENDEKA can cause fetal harm if taken while pregnant. If you are able to become pregnant, your healthcare provider will do a pregnancy test before starting treatment with BENDEKA. Females of reproductive potential should use effective contraception during treatment with BENDEKA and for 6 months after the last dose and for males with female partners for 3 months after the last dose. BENDEKA may also impair male fertility. Females should not breastfeed during treatment with BENDEKA and for 1week after the last dose.
Most common side effects include: fatigue, fever, nausea, and vomiting, diarrhea, constipation, loss of appetite, cough, headache, weight loss, difficulty breathing, rash, mouth irritation, low red blood cells (oxygen-carrying cells), low platelets (blood-clotting cells), and decreased number of three different types of white blood cells (infection-fighting cells).
These are not all of the possible side effects of BENDEKA. For more information ask your healthcare provider.
You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please read the Full Prescribing Information
Approved Use:
BENDEKA is indicated for the treatment of patients with
- ·Chronic lymphocytic leukemia (CLL). Efficacy relative to first-line therapies other than chlorambucil has not been established.
- ·Indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Important Safety Information
BENDEKA is not right for everyone,including patients with a known allergic response to bendamustine, polyethylene glycol 400, propylene glycol, or monothioglycerol.
BENDEKA may cause serious side effects including: low blood cell counts, infections or recurrence of infections, unexpected responses to BENDEKA when placed in your blood, sudden and severe allergic responses, kidney failure due to fast breakdown of cancer cells, other cancers, and leaking of BENDEKA out of your vein and into your surrounding skin. Some of these side effects, such as low blood counts, infections, liver injury, and severe allergic skin responses (when bendamustine HCl was given alone and in combination with other anticancer medications or allopurinol), have caused death.
Tell your doctor if you have any side effects including:
- -Signs of allergic reactions including; rash, facial swelling, or difficulty breathing during or soon after your infusion with BENDEKA injection.
- -Signs of infection including; shortness of breath, significant fatigue, bleeding, bruising, fever, or other signs of infection and or any suspicious skin changes.
- -Confusion, memory loss, trouble thinking, difficulty talking or walking, vision loss or other neurological or cognitive symptoms.
- -Nausea, vomiting, diarrhea, loss of appetite, or a yellow skin tone.
Some serious side effects may require changes in therapy, such as lowering the amount of BENDEKA given, stopping the use of BENDEKA, or waiting longer than expected between doses of BENDEKA.
BENDEKA can cause fetal harm if taken while pregnant. If you are able to become pregnant, your healthcare provider will do a pregnancy test before starting treatment with BENDEKA. Females of reproductive potential should use effective contraception during treatment with BENDEKA and for 6 months after the last dose and for males with female partners for 3 months after the last dose. BENDEKA may also impair male fertility. Females should not breastfeed during treatment with BENDEKA and for 1week after the last dose.
Most common side effects include: fatigue, fever, nausea, and vomiting, diarrhea, constipation, loss of appetite, cough, headache, weight loss, difficulty breathing, rash, mouth irritation, low red blood cells (oxygen-carrying cells), low platelets (blood-clotting cells), and decreased number of three different types of white blood cells (infection-fighting cells).
These are not all of the possible side effects of BENDEKA. For more information ask your healthcare provider.
You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please read the Full Prescribing Information
Contact us
Medical Information
For healthcare professionals or patients with specific medical questions about BENDEKA® (bendamustine HCl) injection, please contact:
Teva Medical Information
1-888-4-TEVARX (1-888-483-8279)
To request more information about BENDEKA, click here
You are about to leave this site
You are about to leave BENDEKA.com and enter a website operated by a third party. Would you like to continue?
Are you a healthcare professional?
The information on this site is intended for healthcare
professsionals
in the United States. Are you a healthcare professional
in the United States?
